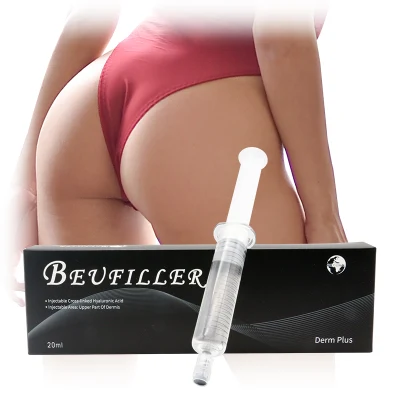
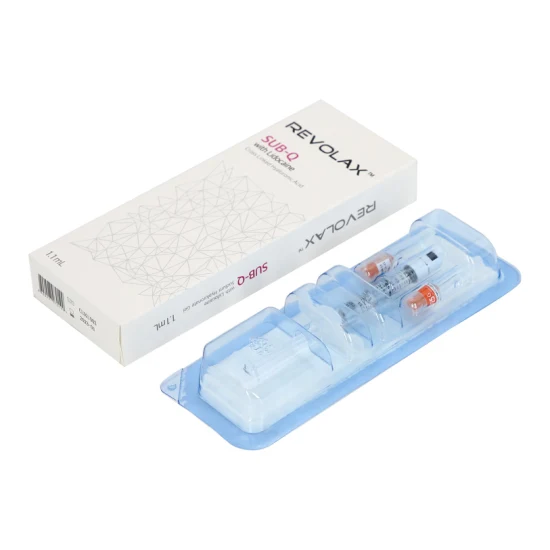
CE Approved Otesaly 2ml Deep Lines Injectable Dermal Filling Cheek Augmentation Hyaluronic Acid Dermal Filler
Overview Product Description About OTESALY® cross-linked Dermal filler √ 1, The raw material of OTESALY cross-linked Der
Send your inquiryDESCRIPTION
Basic Info
| Model NO. | 2ml deep lines |
| Suitable for | Adult |
| Usage Mode | Injection |
| Form | Gel |
| Type | Cross-Linked Dermal Filler |
| Technology | Nasha |
| Composition | 25mg/Ml Cross-Linked Sodium Hyaluronate Gel |
| Injection Areas | Deep Skin Depressions, Nose Bridge, Cheek, Chin. |
| Needle Size | 2PCS *27g |
| Duration | 12-16months |
| Storage | 2-25 Degrees Celsius |
| Transport Package | Box |
| Specification | 2ml deep lines |
| Trademark | OTESALY |
| Origin | China |
Product Description
About OTESALY® cross-linked Dermal filler
√ 1, The raw material of OTESALY cross-linked Dermal filler is imported from USA which
is FDA and Europe EDQM approved.
NASHA means Non-Animal Origin Stabilized Hyaluronic Acid.
√ 2, The Glass Syringes and 2 * 27G Needles are made by B&D Company which is regarded as one
of the best company in medical industry. And the Syringe Plunger is with soft movement. You can move it smoothly during injection.
√ 3, This is Tyvek paper which is anti bacterial and made by Dupont company in USA.
√ 4, The material for the packaging is medical grade PET which is tough enough to protect the filler.
We produce the OTESALY ® dermal filler with same technology as Res--tyl--ane--NASHA, which allows the fillers to last up to 9-12 months.NASHA means Non-Animal Origin Stabilized Hyaluronic Acid technology that creates a stabilized HA for reliable aesthetic treatments with a proven safety record.NASHA creates a precise, firmer gel texture intended for a more pronounced lift and targeted product integration.
★ High density:Stable and long-lasting, without adding a large number of links.★ Non-animal: It is fermented by bacteria, and its ingredients are very similar to hyaluronic acid in the human body, so it is really safe and suitable for all skin types.★ Non-permanent: It can be maintained for an average of about 9-12 months, and gradually decomposes into carbon dioxide and water in the body, so as to be excreted.
The particle size of each type of dermal filler is different. So it makes that they can be injected in different area of face or body with different results.
1. Derm Lines, It can be injected on the treatment of fine wrinkles on face such as lip lines, frown lines, crow's feet, forehead lines. It also can fill the hollow area of under eyes and give volume to the lips.2, Deep Lines, It can be injected on the treatment of wrinkles and deep skin depressions. it provide lift and enhance the facial contours such as deep skin depressions, nose bridge, cheek, chin, jawline, and temples. 3, Deep lines with Lido, It can be injected on the treatment of wrinkles and deep skin depressions. it provide lift and enhance the facial contours such as deep skin depressions, nose bridge, cheek, chin, jawline, and temples. 4, Derm Plus, it can be injected on breast and buttock.First let US show you OTESALY 2ml deep lines cross-linked dermal filler
| Type | OTESALY®2ml deep lines |
| Composition | 25mg/ml cross-linked sodium hyaluronate gel |
| Approximate number of gel particles 1ml | 10,000 |
| Injection areas | It can be injected on the treatment of wrinkles and deep skin depressions. it provide lift and enhance the facial contours such as nose bridge, cheek, chin, jawline, and temples. |
| Where to inject | Dermis |
| Volume of syringe | 2ml |
| Needle size | 2pcs *27G |
| Duration | 12-16months |
| Storage | 2-25 Degrees Celsius |
Detailed Photos
1, Main products
Our product lines include Cross-linked dermal filler; bo- tox(50iu, 100iu, 150iu); Mesotherapy solution from skin rejuvenation, skin Whitening, Fat dissolving injection, Hair growth injection,etc.
2, Trademark
Our trade mark are OTESALY and SOMED, which are registered in Russia in USA, Europe, Russia, Kazakhstan, Iraq, Brazil, Mexico, Colombia,etc.
3, 16 Years History
Aoma CO.,LTD was established in 2005, covers an area of 4,800 square meters of factory, 3 production lines, started export medical products since 2006.
We are verified supplier on Alibaba for more than 10 years with good reputation.
4, GMP workshop
Our factory covers an area of 4,800 square meters, founded with 800 square meters 10,000 class purification workshop and 300 square meters 100 class purification workshop. The production area is about 3,200 square meters. we have a terminal sterilization technology and strict quality control system,The sodium hyaluronate gel is sterilized by high pressure steam,and the needle is sterilized by gamma rays.The preparation is aseptic and pyrogen-free,which ensure the safety and high quality of the products without pollution.
5, Top production equipment
We have the most advanced production equipment imported from Europe countries,such as Automatic vacuum filling and stoppering machine from Germany OPTIMA, two-door cabinet type sterilizer from Sweden GETINGE, Agilent HPLC, UV, Shimadzu GC, Malvern rheometer, etc.
6, Strict clinical test
We entered the clinical test from 2006, and cooperates with medical institutions such as The First Affiliated Hospital of Zhejiang University, Shanghai Ninth People's Hospital,etc. The results show that our cross-linked sodium hyaluronate gel for plastic surgery can meet the clinical needs,the quality of the prepared products is stable, the filling effect is good, the maintenance time is long, and the rate of adverse reactions is low.
7, CE, ISO 13485 CERTIFICATE.
AOMA CO.,LTD import the F-D-A & Europe EDQM approved raw material of Medical Sodium Hyaluronate Gel which costs around 45,000USD/ kg from USA. is the earliest product in China that has obtained dual certification of CE and international GMP certification. More than 4 million people worldwide have been used AOMA CO.,LTD products so far. 96% of customers buy again after tried AOMA CO.,LTD products.
Related Products
-
![Disposable Medical Supplies Y Type Safety Huber Needles]()
Disposable Medical Supplies Y Type Safety Huber Needles
-
![19g 100mm Skin Tightening Barbed Pdo Molding Cog Thread for Face Lifting]()
19g 100mm Skin Tightening Barbed Pdo Molding Cog Thread for Face Lifting
-
![Injectable Hyaluronic Acid Gel Injection 1ml Lip Dermal Filler Hydrogel Injections Deep Pen Perfecta]()
Injectable Hyaluronic Acid Gel Injection 1ml Lip Dermal Filler Hydrogel Injections Deep Pen Perfecta
-
![1ml 2ml 10ml 20ml Hyaluronic Acid Dermal Filler Injection Fine/Derm/Deep Line Ha Derm Filler]()
1ml 2ml 10ml 20ml Hyaluronic Acid Dermal Filler Injection Fine/Derm/Deep Line Ha Derm Filler